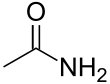
Acetamide
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetamide[1] | |||
| Systematic IUPAC name
Ethanamide | |||
| Other names
Acetic acid amide
Acetylamine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.430 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5NO | |||
| Molar mass | 59.068 g·mol−1 | ||
| Appearance | colorless, hygroscopic solid | ||
| Odor | odorless mouse-like with impurities | ||
| Density | 1.159 g cm−3 | ||
| Melting point | 79 to 81 °C (174 to 178 °F; 352 to 354 K) | ||
| Boiling point | 221.2 °C (430.2 °F; 494.3 K) (decomposes) | ||
| 2000 g L−1[2] | |||
| Solubility | ethanol 500 g L−1[2] pyridine 166.67 g L−1[2] soluble in chloroform, glycerol, benzene[2] | ||
| log P | −1.26 | ||
| Vapor pressure | 1.3 Pa | ||
| Acidity (pKa) | 15.1 (25 °C, H2O)[3] | ||
| −0.577 × 10−6 cm3 g−1 | |||
Refractive index (nD)
|
1.4274 | ||
| Viscosity | 2.052 cP (91 °C) | ||
| Structure | |||
| trigonal | |||
| Thermochemistry[4] | |||
Heat capacity (C)
|
91.3 J·mol−1·K−1 | ||
Std molar
entropy (S⦵298) |
115.0 J·mol−1·K−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−317.0 kJ·mol−1 | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H351 | |||
| P201, P202, P281, P308+P313, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 126 °C (259 °F; 399 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
7000 mg kg−1 (rat, oral) | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is an amide derived from ammonia and acetic acid. It finds some use as a plasticizer and as an industrial solvent.[5] The related compound N,N-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide. Acetamide can be considered an intermediate between acetone, which has two methyl (CH3) groups either side of the carbonyl (CO), and urea which has two amide (NH2) groups in those locations. Acetamide is also a naturally occurring mineral[6] with the IMA symbol: Ace.[7]
Production
[edit]
Laboratory scale
[edit]Acetamide can be produced in the laboratory from ammonium acetate by dehydration:[9]
- [NH4][CH3CO2] → CH3C(O)NH2 + H2O
Alternatively acetamide can be obtained in excellent yield via ammonolysis of acetylacetone under conditions commonly used in reductive amination.[10]
It can also be made from anhydrous acetic acid, acetonitrile and very well dried hydrogen chloride gas, using an ice bath, alongside more valuable reagent acetyl chloride. Yield is typically low (up to 35%), and the acetamide made this way is generated as a salt with HCl.
Industrial scale
[edit]In a similar fashion to some laboratory methods, acetamide is produced by dehydrating ammonium acetate or via the hydration of acetonitrile, a byproduct of the production of acrylonitrile:[5]
- CH3CN + H2O → CH3C(O)NH2
Uses
[edit]Acetamide is used as a plasticizer and an industrial solvent.[5] Molten acetamide is good solvent with a broad range of applicability. Notably, its dielectric constant is higher than most organic solvents, allowing it to dissolve inorganic compounds with solubilities closely analogous to that of water.[11] Acetamide has uses in electrochemistry and the organic synthesis of pharmaceuticals, pesticides, and antioxidants for plastics.[12] It is a precursor to thioacetamide.[13]
Occurrence
[edit]Acetamide has been detected near the center of the Milky Way galaxy.[14] This finding is potentially significant because acetamide has an amide bond, similar to the essential bond between amino acids in proteins. This finding lends support to the theory that organic molecules that can lead to life (as we know it on Earth) can form in space.
On 30 July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67/P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which – acetamide, acetone, methyl isocyanate, and propionaldehyde[15][16][17] – were seen for the first time on a comet.
In addition, acetamide is found infrequently on burning coal dumps, as a mineral of the same name.[18][19]
References
[edit]- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 841. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d The Merck Index, 14th Edition, 36
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–88. ISBN 9781498754293.
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–3. ISBN 978-1138561632.
- ^ a b c Cheung, H.; Tanke, R. S.; Torrence, G. P. "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.pub2. ISBN 978-3527306732.
- ^ Mindat: Naturally occurring acetamide
- ^ Warr, L.N. (2021). "IMA-CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Bats, Jan W.; Haberecht, Monika C.; Wagner, Matthias (2003). "A new refinement of the orthorhombic polymorph of acetamide". Acta Crystallographica Section E. 59 (10): o1483–o1485. doi:10.1107/S1600536803019494.
- ^ Coleman, G. H.; Alvarado, A. M. (1923). "Acetamide". Organic Syntheses. 3: 3. doi:10.15227/orgsyn.003.0003; Collected Volumes, vol. 1, p. 3.
- ^ Schwoegler, Edward J.; Adkins, Homer (1939). "Preparation of Certain Amines". J. Am. Chem. Soc. 61 (12): 3499–3502. doi:10.1021/ja01267a081.
- ^ Stafford, O. F. (1933). "Acetamide as a Solvent". J. Am. Chem. Soc. 55 (10): 3987–3988. doi:10.1021/ja01337a011.
- ^ Wagner, Frank S. (2002). Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons. doi:10.1002/0471238961.0103052023010714.a02.pub2. ISBN 9780471238966.
- ^ Schwarz, G. (1945). "2,4-Dimethylthiazole". Organic Syntheses. 25: 35; Collected Volumes, vol. 3, p. 332.
- ^ Hollis, J. M.; Lovas, F. J.; Remijan, A. J.; Jewell, P. R.; Ilyushin, V. V.; Kleiner, I. (2006). "Detection of Acetamide (CH3CONH2): The Largest Interstellar Molecule with a Peptide Bond". Astrophys. J. 643 (1): L25–L28. Bibcode:2006ApJ...643L..25H. doi:10.1086/505110.
- ^ Jordans, Frank (30 July 2015). "Philae probe finds evidence that comets can be cosmic labs". The Washington Post. Associated Press. Archived from the original on 23 December 2018. Retrieved 30 July 2015.
- ^ "Science on the Surface of a Comet". European Space Agency. 30 July 2015. Retrieved 30 July 2015.
- ^ Bibring, J.-P.; Taylor, M.G.G.T.; Alexander, C.; Auster, U.; Biele, J.; Finzi, A. Ercoli; Goesmann, F.; Klingehoefer, G.; Kofman, W.; Mottola, S.; Seidenstiker, K.J.; Spohn, T.; Wright, I. (31 July 2015). "Philae's First Days on the Comet - Introduction to Special Issue". Science. 349 (6247): 493. Bibcode:2015Sci...349..493B. doi:10.1126/science.aac5116. PMID 26228139.
- ^ "Acetamide". Mindat.org.
- ^ "Acetamide" (PDF). Handbook of Mineralogy. RRUFF Project.
External links
[edit]- International Chemical Safety Card 0233
- "Acetamide". Webmineral.org.